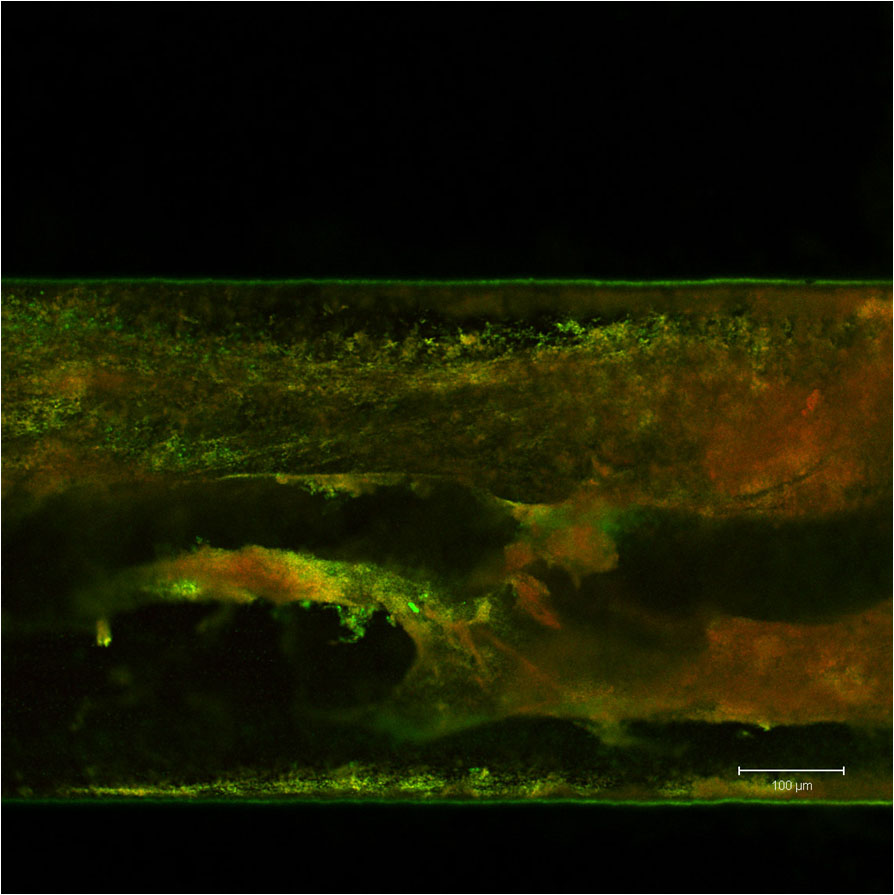
 Bacterial biofilm formed in a microfluidic channel.
Bacterial biofilm formed in a microfluidic channel. Certain types of bacteria regulate gene expression through quorum-sensing, the detection of extracellular levels of signaling compounds produced by bacteria of the same species. Once bacteria such as E. coli sense their population is sufficiently large to overwhelm a host’s immune system, they will aggregate and form a pathogenic matrix of bacteria, or biofilm. Biofilms characterize the majority of clinical infections, and are difficult to eliminate once formed.
My research focuses on developing microfluidic platforms for investigating biofilm growth, with the aim of helping scientists develop new anti-biotilm treatments. Using microscale platforms eliminates the need for using large volumes of stains and drugs, and also allows for integrating novel sensing technologies. Some of my previous work focused on verifying the use of optical density as a means to continuously evaluate changes in biofilm formation, gathering the same information provided by a microscope but only using a set of LEDs and photodiodes. This platform was used to verify biofilm reduction by new quorum-sensing inhibitors developed by collaborators.
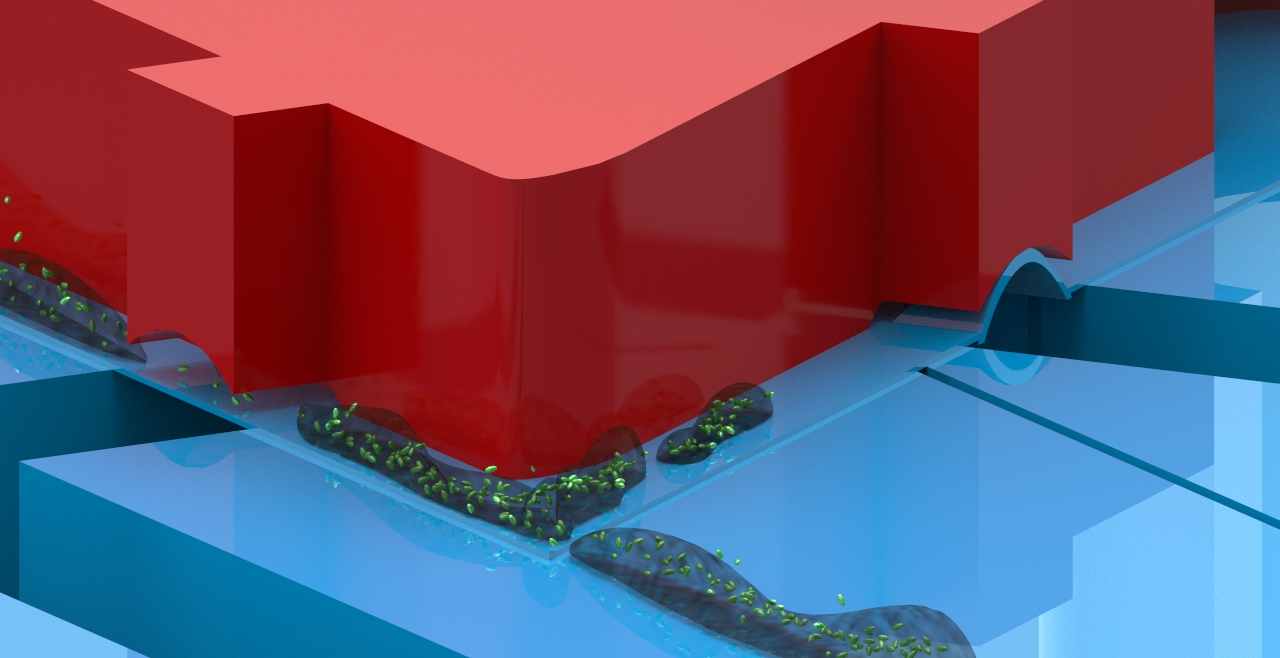
Schematic of microfluidic platform for biofilm segmentation. I am working on developing an microfluidic platform to help create more controlled biofilm assays. This involves growing bacteria under flow in a microfluidic channel, then creating discrete sections of biofilm using integrated pneumatic valves. The methodology helps integrate more assays per device, and compensates for device-to-device variation.
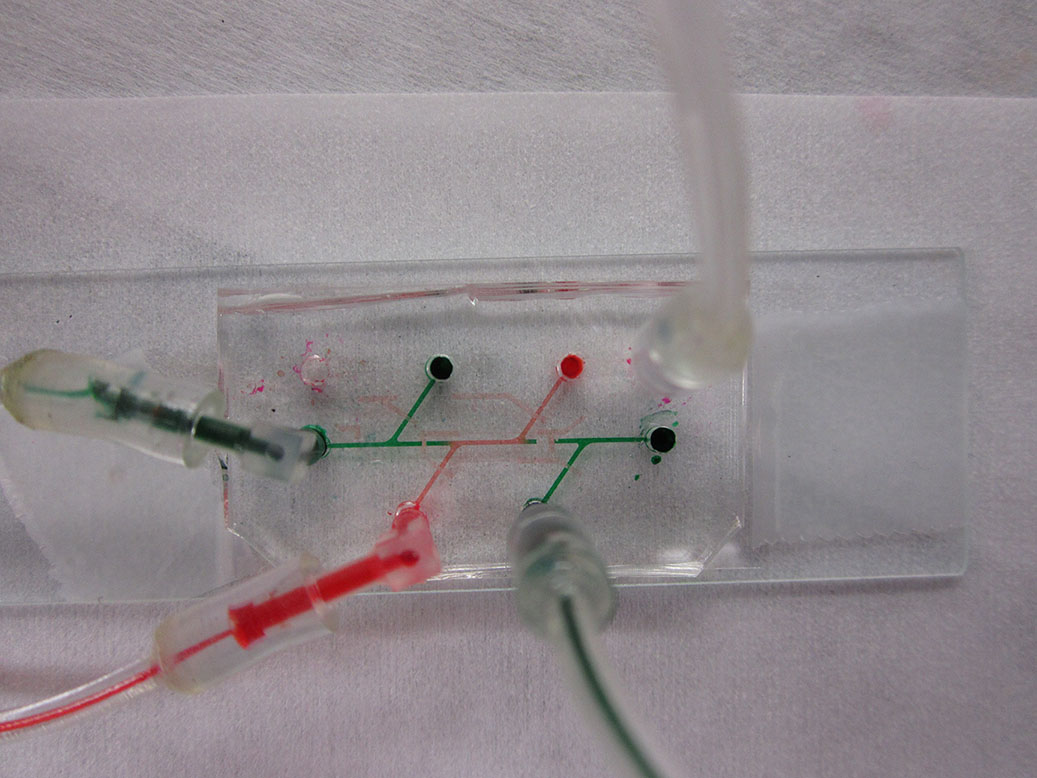
Microfluidic device with integrated pneumatic valves.
Read more about my research:
New Microfluidic Device Could Speed Drug Evaluation
Preventing Costly, Life-Threatening Catheter Infections